Hard Water & What You Need To Know
A blog co-written by MC Web Studio
Hard water is simply defined as water that contains calcium and magnesium minerals. There are usually a lot of undesirable effects when calcium and magnesium are dissolved in water. It can harm hair and irritate the skin, along with annoying effects.
What Is Hard Water? Let Us Explain
As we’ve established, the mineral build up of elements such as calcium and magnesium in water causes hardness.
Calcium deposits naturally occur when water comes into touch with calcium-containing minerals, sedimentary rock, and limestone. It builds up in the water as it passes through the limestone material, hardening it.
Magnesium deposits are present in large quantities in rocks, including dolomite. As a result, the water picks some magnesium up as it passes over these rocks, causing hardness.
Hard water can shorten the lifespan of your dishwasher, clothes washer, and other water-using appliances by causing excessive scale formations or scale deposits in pipes and equipment. The scale could be white and is unlikely to wipe off readily.
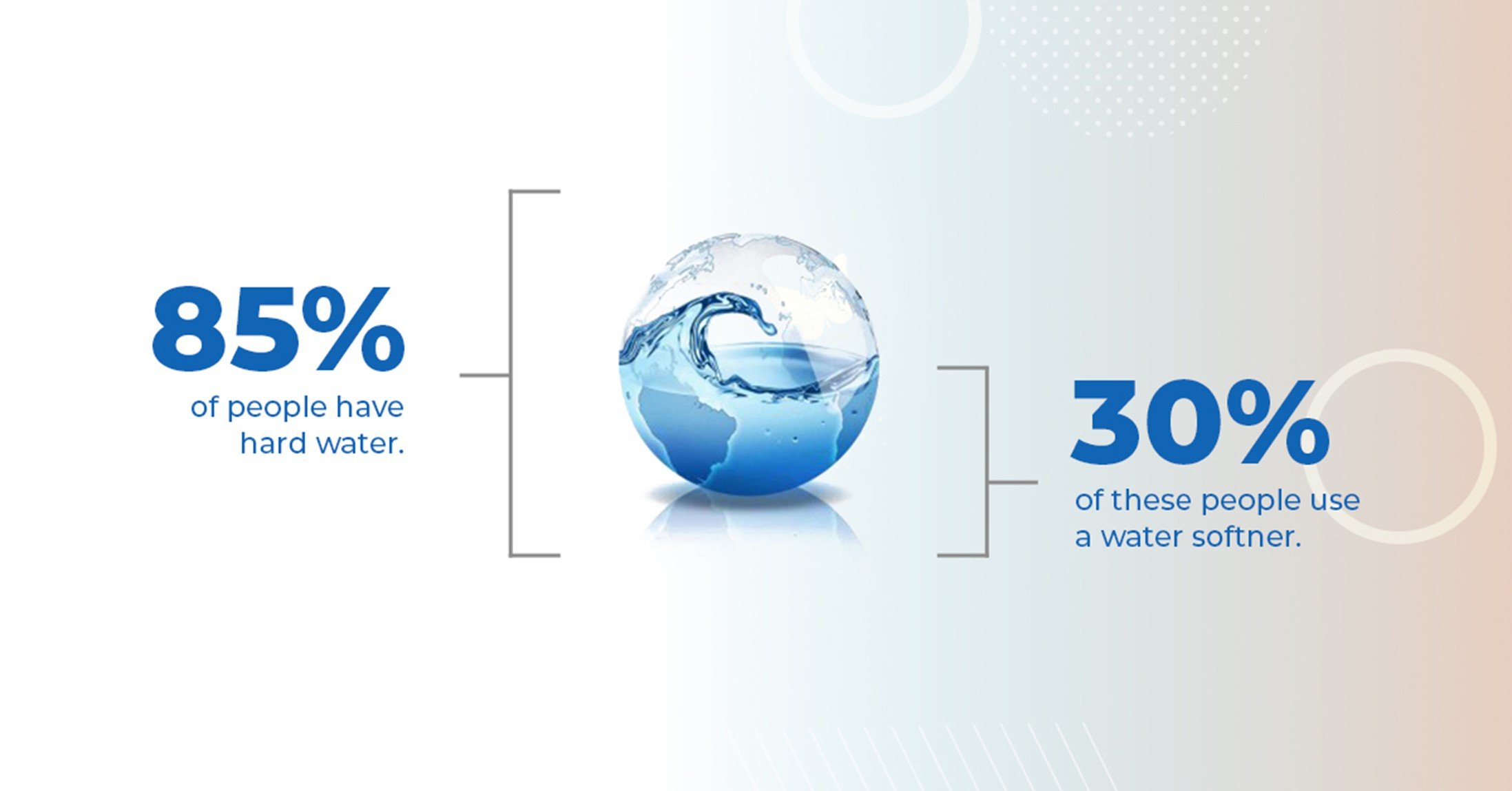
More than 8 out of 10 households face the problem of hard water with mineral-rich water that contains carbonates of calcium and magnesium. While most individuals may adequately consume these minerals, hard water can be highly inconvenient daily.
Hard Water Signs & What You Need To Look For
Harsh Laundry
Hard water minerals like iron and calcium can react with the chemicals in detergent, lowering its efficacy and prematurely fading the colors of your clothes. Even little chalky or rusty stains or an uncomfortable stiffness or itchiness in the cloth are possible effects of hard water.
Stained Dishes
Your dishes may also appear dirty if you use hard water. It's probably not leftover food if you've observed that water glasses or plates have specks or stains on them after they've been in the dishwasher. It's curd, a mixture of detergent and minerals buildup brought on by hard water.
Difficulty Cleaning Bathtubs
Any bathtub or sink often used will require routine cleaning to remove typical soap scum. However, limescale deposits caused by hard water are highly resistant to cleaning agents. Be prepared to put in a lot of extra effort when you clean.
Hair Damage
In addition to causing residue on your scalp and reducing your hair's natural shine, hard water can also deposit minerals in your hair. By preventing moisture from entering your hair shafts and drying them out, the deposits may cause double harm. The issue might get worse by just shampooing more or washing for longer.
Clogs In The Plumbing
Your pipes likely contain deposits if you see chalky buildup on the inside of your faucets. This accumulation of limescale over time can reduce water flow, clog equipment like water heaters, and obstruct shower heads.
Water Hardness Scale
A recommended maximum level for hardness hasn't been identified since people's perceptions of hardness might vary greatly depending on the local environment. Hardness levels above 200 mg/L are regarded as bad but have been tolerated by customers; those above 500 mg/L are undesirable for most residential uses.
Hardness is often reported as equivalent concentration levels of calcium carbonate in ppm. It may be determined by the interaction of polyvalent metallic ions in a water sample with a chelating agent like ethylenediaminetetraacetic acid (EDTA).
According to the amount of calcium carbonate present, drinking water hardness can be categorized as follows: 0 to 60 mg/L is mild; 60 to 120 mg/L is medium hard; 120 to 180 mg/L is hard, and 180 mg/L and beyond is extremely hard.
In a 1975–1977 nationwide study of Canadian surface waterways, the following range of average hardness levels was determined for each station: Alberta: 98 to 329 mg/L; Saskatchewan: 12 to 132 mg/L; Manitoba: 15 to 716 mg/L; British Columbia: 7 to 180 mg/L; Northwest Territories: 5 to 179 mg/L. Provinces in the Maritime were not monitored for hardness levels. Water hardness in the upper Great Lakes ranged from 40 to 80 mg/L.
Hardness levels in Ontario lakes and streams varied greatly; amounts as high as 1803 mg/L were reported, although the majority were between 40 and 200 mg/L. 41 areas were chosen as indicative of Canadian waterways to evaluate the country's water quality.
Except for the Nelson-Saskatchewan and Mississippi basins, the median of the amounts observed at each site hardly surpassed 120 mg/L. Most hardness ratings in these rivers and streams exceed 180 mg/L. Hence the waters are thought to be hard. None of the 41 stations' median values were more than 500 mg/L.
Half of all Canadian towns had water hardness levels under 80 mg/L, while 20% had levels over 180 mg/L, according to a study of municipal water systems across the country. Levels only significantly exceeded 180 mg/L in the Prairie provinces and Ontario.
In Ontario, drinking water from surface sources had a hardness that varied from 3.7 to 296 mg/L, with a typical of 95 mg/L; groundwater supplies had a harder hardness that ranged from 40 to 1300 mg/L, with an aggregate of 294 mg/L. Only 17 Canadian communities reported potable water hardness levels exceeding 500 mg/L, according to a recent assessment of 525 municipalities. Both Ontario and Saskatchewan contained these cities.
Hardest Water In Canada – Where Does British Columbia Fit In?
In Canada, 20% of municipal water sources had water hardness levels below 80 mg/L. On the other hand, you might find water hardness levels substantially greater than 180 mg/L in Ontario and the Prairie provinces.
The findings of groundwater sources taken as part of the Water Quality Inspection Program between 1977 and 1993 were assessed by the Ministry of the Environment. Hardness was analyzed in over 12,000 samples, with 5.4% exhibiting hardness values of more than 500 mg/L.
Additionally, 10% of samples had hardness between 120 mg/L and 180 mg/L, 22% had hardness equal to or greater than 180 mg/L, and 40% of samples had a hardness of less than 60 mg/L. In general, igneous rock formations produce soft water, whereas sedimentary rock formations produce more hard water. British Columbia has some of the hardest water areas compared to other territories or provinces.
Source: https://city.langley.bc.ca/city-services/engineering-parks-operations/water
Hard Water In Langley Township, British Columbia
British Columbia Drinking Water Protection states that all water suppliers submit a yearly report on groundwater quality and, ensuring that data is available to the public, governs how groundwater quality reports are created.
Residents who aren't connected to the township's water supply get their water from personal or public wells, 17 wells distribute water to the Township of Langley by GVWD.
Hard water is common in this area due to the breakdown of minerals in the water as it travels through the soils and rock. Down below are some ways to prevent hard water and how to make it more usable for everyday life.
A graph showing water hardness levels in Langley Township wells.
How To Prevent Hard Water
Boiling
Boiling is the most convenient and preferred method of cleaning hard water. It is a simple process where water is heated until boiling point. This temperature helps kill bacteria and remove some minerals that cause hardness. In this process, however, some more complex compounds may be left behind that can only be removed by distillation.
Distillation
In distillation, water is heated to its boiling point until it completely vaporizes. This vapor is collected and introduced to a condenser for cooling. As it cools, the vapor transforms into pure liquid water, free of all minerals and microorganisms, even essential ones. Substances with a more extreme boiling point are left as sediments in the container.
Filtration
Water filtration helps remove dangerous contaminants that cause diseases. It is one of the most productive methods for removing pollutants from hard water. This process relies on physical and chemical means to make water healthy for human consumption.
Chlorination
Chlorination is a powerful water purification method that efficiently removes germs and parasites that may be detected in the tap or subsurface. Water may be cleaned with liquid or tablet chlorine, but only controlled amounts should be used.
Conclusion
Hard water is rich in minerals, but not in a good way. Excessive amounts of calcium, magnesium, etc., cause harm in several ways. It cannot be used domestically and might damage your hair and skin. This is why it needs to be treated before being released into pipes. If you suspect hard water contamination in your pipes, contact Lucid Water to learn about their water filtration services!
Hard Water Frequently Asked Questions
How do you know if your water is hard?
Fill the bottle up to one-third complete, add only a few droplets of clean laundry detergent, and shake it violently for a few seconds. If there are no frothy froths and the water seems whitish.
What is the difference between hard water and soft water?
While soft water lacks abrasive elements that might harm your health and house, hard water has excessive amounts of calcium and magnesium. Through water softener systems, calcium and magnesium are gently removed.
Is hard water okay to drink?
Regarding cardiovascular disease, calcium and magnesium in drinking water have a dose-dependent preventive impact. As a result, you can not drink hard water.
Is Langley tap water safe to drink?
Yes, the standards for drinking water quality established by the BC Drinking Water Protection Regulation were satisfied by the City in 2021. E. coli was not detected in any of the samples. No total coliform bacteria are present in 100 ml.